Revision Notes on Plant Growth and Development
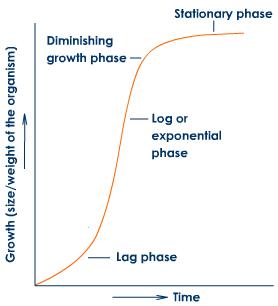
(1) The analysis of growth curve shows that it can be differentiated into three phases:
(i) Lag phase: It represents initial stages of growth. The rate of growth is very slow in lag phase. More time is needed for little growth in this phase.
(ii) Log phase (Exponential phase): The growth rate becomes maximum and more rapid. Physiological activities of cells are at their maximum. The log phase is also referred to as grand period of growth.
(iii) Final steady state (Stationary phase) or Adult phase: When the nutrients become limiting, growth slows down, so physiological activities of cells also slows down. This phase is indicated by the maturity of growth system. The rate of growth can be measured by an increase in size or area of an organ of plant like leaf, flower, fruit etc. The rate of growth is called efficiency index.
(2) Phytohormones:-
(i) Growth hormones also called phytohormones
(ii) Term given by Thimann (1948),
(iii) It can be defined as ‘the organic substances which are synthesized in minute quantities in one part of the plant body and transported to another part where they influence specific physiological processes’.
Growth Hormones and Growth Regulators
(1) Auxins:
(i) Auxins (Gk. auxein = to grow) are weakly acidic growth hormones having an unsaturated ring structure and capable of promoting cell elongation, especially of shoots (more pronounced in decapitated shoots and shoot segments) at a concentration of less than 100 ppm which is inhibitory to the roots. Among the growth regulators, auxins were the first to be discovered.
(ii) Types of auxins: There are two major categories of auxins natural auxins and synthetic auxins:
(a) Natural auxins: These are naturally occurring auxins in plants and therefore, regarded as phytohormones. Indole 3-acetic acid (IAA) is the best known and universal auxin. It is found in all plants and fungi.
(b) Synthetic auxins: These are synthetic compounds which cause various physiological responses common to IAA. Some of the important synthetic auxins are 2, 4-D (2, 4-dichlorophenoxy acetic acid) is the weedicide. IBA is both natural and synthetic auxin.
(iii) Functions of auxins: Auxins control several kinds of plant growth processes. These are as follows:
(a) Cell elongation: Auxins promote elongations and growth of stems and roots and enlargement of many fruits by stimulating elongation of cells in all directions.
(b) Apical dominance: In many plants, the apical bud grows and the lower axillary buds are suppressed. Removal of apical bud results in the growth of lower buds. The auxin (IAA) of the terminal bud inhibits the growth of lateral buds. This phenomenon is known as apical dominance.
(c) Weed control: Weeds are undesirable in a field with a crop. By the spray of 2, 4-D, broad-leaved weeds can be destroyed but 2, 4-D does not affect mature monocotyledonous plants.
(d) Root differentiation
(e) Control of lodging
(f) Parthenocarpy: Parthenocarpy can be induced by application of IAA in a paste form to the stigma of a flower or by spraying the flowers with a dilute solution of IAA.
(2) Gibberellins:
(i) Gibberellins are weakly acidic hormones having gibbane ring structure which cause cell elongation of intact plants in general and increased internodal length of genetically dwarfed plants (i.e., corn, pea) in particular.
(ii) Functions of gibberellin
(a) Stem elongation: The gibberellins induce elongation of the internodes.
(b) Leaf expansion: In many plants leaves become broader and elongated when treated with gibberellic acid.
(c) Reversal of dwarfism: One of the most striking effects of gibberellins is the elongation of genetic dwarf (mutant) varieties of plants like corn and pea.
(d) Bolting and Flowering: Gibberellins induce stem elongation in ‘rosette plants’ e.g., cabbage, henbane, etc. Such plants show retarded internodal growth and profuse leaf development. In these plants just prior to the reproductive phase, the internodes elongate enormously causing a marked increase in stem height. This is called bolting.
(e) Enzyme formation: One of the most dramatic effects of GA is its induction of hydrolytic enzymes in the aleurone layer of endosperm of germinating barley seeds and cereal grains. GA stimulates the production of digestive enzymes like proteases, a-amylases, lipases which help to mobilise stored nutrients.
(f) Breaking of dormancy: Gibberellins overcome the natural dormancy of buds, tubers, seeds, etc. and allow then to grow. In this function, gibberellins act antagonistically to abscisic acid (ABA).
(g) Parthenocarpy: Gibberellins have been considered to be more effective than auxins for inducing parthenocarpy in fruits like apple, tomato and pear. GA application has also resulted in the production of large fruits and bunch length in seedless grapes.
(h) Sex expression: Gibberellins control sex expression in certain plants. In general, gibberellin promotes the formation of male flowers either in place of female flowers in monoecious plants such as cucurbits or in genetically female plants like Cannabis, Cucumis.
(3) Cytokinins (Phytokinins):
(i) Cytokinins are plant growth hormones which are basic in nature, either aminopurine or phenyl urea derivatives that promote cell division (cytokinesis) either alone or in conjugation with auxin.
(ii) Functions of cytokinins
(a) Cell division: Cytokinins are essential for cytokinesis and thus promote cell division. In presence of auxin, cytokinins stimulate cell division even in non-meristematic tissues.
(b) Cell enlargement and Differentiation: Under some conditions cytokinins enhance the expansion of leaf cells in leaf discs and cotyledons. These cells considered to be mature and under normal conditions do not expand.
(c) Delay in senescence: Cytokinin delay the senescence (ageing) of leaves and other organs by controlling protein synthesis and mobilization of resources (Disappearance of chlorophyll). It is called Richmond Lang effect.
(d) Counteraction of apical dominance: Auxins and cytokinins act antagonistically in the control of apical dominance. Auxins are responsible for stimulating growth of apical bud.
(e) Breaking of dormancy: Cytokinins breaks seeds dormancy of various types and thus help in their germination.
(f) Accumulation and Translocation of solutes
(4) Ethylene:
(i) Ethylene is a gaseous hormone which stimulates transverse growth but retards the longitudinal one.
(ii) Functions of ethylene
(a) Fruit growth and Ripening: Ethylene promotes fruit growth and its ripening. The harmone is used in the artificial ripening of climacteric fruits (e.g., Apple, Banana, and Mango).
(b) Transverse growth: Ethylene inhibits longitudinal growth but stimulates transverse growth so that stem looks swollen.
(c) Epinasty (leaf bending): Epinasty represents more growth on upper surface of leaf than on lower surface. Epinasty is said to be controlled by ethylene in many plants.
(d) Abscission: Ethylene stimulates formation of abscission zone in leaves, flowers and fruits.
(e) Apical dominance: Ethylene inhibits the growth of lateral buds and thus causes apical dominance (in pea). It is believed that auxin might be functioning partly through synthesis of ethylene in causing apical dominance.
(f) Root initiation: In low concentration, ethylene stimulates root initiation and growth of lateral roots and root hair.
(g) Flowering: Ethylene stimulates flowering in pineapple and related plants though in other cases, the hormone causes fading of flowers.
(5) Abscisic acid (ABA):
(i) Abscisic acid is a mildly acidic growth hormone, which functions as a general growth inhibitor by counteracting other hormones (auxin, gibberellins, cytokinins) or reactions mediated by them.
(ii) Functions of abscisic acid
(a) Control: It keeps growth under check by counter acting the effect of growth promoting hormones, i.e., auxins, cytokinins and gibberellins. As growth is primarily controlled by gibberellins, abscisic acid is popularly called antigibberellic hormone.
(b) Dormancy: Abscisic acid acts as growth inhibitor and induces dormancy of buds towards the approach of winter.
(c) Abscission: ABA promotes the abscission of leaves, flowers and fruits in plants.
(d) Senescence: Abscisic acid stimulates senescence of leaves by causing destruction of chlorophyll (an effect opposite to that of cytokinins) and inhibition of protein and RNA synthesis.